Fantastic Caytoniales and how to reconstruct them.
 |
Fig. 1 Life Reconstructions of Selected Caytoniales. |
Greetings
all,
It’s
been over two years now since I last wrote a blog post, so I’m sorry about
that. I probably could have written something at some point, but uni work or
something else has always gotten in the way… I’m also pretty lazy so that
doesn’t really help either. Anyway, I have been prompted into writing again by
a recent post on twitter (see here) where the question was asked, “Do we have
any headway on how to reconstruct Caytoniales as an entire plant?” I have been
asked this same question a number of times this last year, and since there are
some reasonably good answers to give (as well as some uncertainties to
discuss), I thought I’d cover it in a blog post.
And so here we are, I shall now
impart onto you the most sacred and glorious knowledge of how to reconstruct
Caytoniales!!! [cue the dramatic fanfare]
Meet the Caytoniales
So firstly, what are the Caytoniales? Well, they’re a family of plants which was first established by H. H. Thomas almost 100 years ago in 1925, based on material collected from the Gristhorpe Plant Bed of Cayton Bay, Yorkshire, England. This material consisted of female fruiting bodies (Gristhorpia nathorsti and Caytonia sewardi; although Gristhorpia was later synonymised with Caytonia in Harris, 1940), male ‘flowering structures’ (Antholithus arberi; later renamed Caytonanthus by Harris, 1941) and palmate leaves with anastomosing venation (Sagenopteris) (Harris, 1964) (Fig. 2). Thomas concluded, based on the continuous association of these three organs at various fossil sites, that these organs all came from a single type of plant which he described as being a basal angiosperm. Subsequent
 |
Fig. 2 Whole Plant Hypotheses for different caytoniale taxa. |
find from across the world have confirmed this hypothesised Caytonia plant (Fig. 3) (although only the leaves Sagenopteris have been found in North America) (Harris, 1964; Taylor et al., 2009), but the affinities of this plant are less certain. The Caytoniales are seed ferns, however, this doesn’t help us as ‘seed fern’ is a catch-all term for any fossil plant with fern-like foliage which also produces seeds and is composed of many different clades of unrelated plants. Phylogenetic analyses generally place the Caytoniales as being a sister group to angiosperms (within the Angiophytes; Crane, 1985; Shi et al., 2021), which is supported morphologically by similarity of their cupules to the fruits of angiosperms, and their angiosperm-like leaves (Harris, 1964).
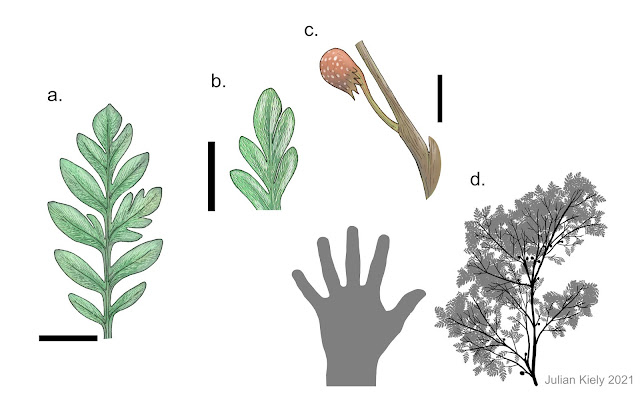
Several other genera of Caytoniales have now been described, from both floral and reproductive remains. A Caytonia-like organ named Reymanownaea kvacekii, which is associated with Sagenopteris and Caytonanthus remains, was found in the Lower Jurassic Karolinavölgy Formation of Hungary (Fig. 4) (Barbacka and Bóka, 2000a). Several taxa are known from South America, although not all of these have been fully described. The most well known of these is Ruflorinia; three species are known from the Aptian aged Baqueró Group (R. sierra, R. pilifera and R. papillosa) and a fourth species hails from the Berriasian–Valanginian aged Springhill Formation (R. orlandoi) (Carrizo et al., 2014). The leaves of this genus are bipinnatifid and fern-like (Fig. 8) and quite unlike the leaves of Sagenopteris, however they are assigned to the Caytoniales based on the association of R. sierra with the female reproductive
organ Ktalenia circularis, which is very similar structurally to Caytonia (although it is visually rather different; Fig. 4) (Taylor and Archangelsky, 1985). An unnamed taxon of plant from the Aptian aged Crato Formation of Brazil, affectionately called ‘The Crato Seed Fern’, possesses Caytonia-like reproductive organs and likely belongs to the Caytoniales (Mohr et al., 2007), however it is awaiting a systematic description and is currently enigmatic (Fig. 7). The isolated leaf morphogenera Scoresbya and Mexiglossa are similar to Sagenopteris, and thus may also be Caytoniales, however without associated reproductive organs their affinities cannot be confirmed (Taylor et al., 2009; Retallack and Dilcher, 1988). Isolated seeds of the type found in Caytonia are placed in the morphogenus Amphorispermum, and pollen grains from Caytonanthus are included in Vitreisporites (Taylor et al., 2009).
The Caytoniales existed throughout most of the Mesozoic, from the Late Triassic until the Albian stage of the Early Cretaceous (Miller and Hickey, 2010; Taylor et al., 2009) and had a near global distribution, colonising every continent with the exception of Africa, and were most widespread during the Jurassic (Prakash and Das, 2017). Some taxa, such as the various organs of the Caytonia plant, are found throughout this range, however other taxa had restricted distributions; Ruflorinia was apparently restricted to Patagonia (Carrizo et al., 2014), and Reymanownaea and the Crato taxon are thus far unique to their respective formations.
Reconstructing the whole
plant
Ahh,
I see you’ve made it past the tedious part filled with taxonomic confusion. Now
for the fun part; how to actually reconstruct these plants as whole living
organisms!!! I will break down the following section into several parts, so
that I can cover the various types of plants here separately.
A brief* note on the Whole
Plant Concept
As
you may have noticed (and been rightly confused by) the Caytonia plant
is composed of three different genera (well, actually five if you include seed
and pollen taxa). Unlike in animals, where a single name is used for a single
biological species, within palaeobotany the nature of plant preservation forces
us to assign different names to different organs of a plant. Plants are rarely
preserved whole, so instead it is more convenient to name individual organs of
a plant; the female reproductive organs are given a name (e.g. Caytonia),
the male reproductive organs are given a different name (e.g. Caytonanthus),
the leaves are given another name (e.g. Sagenopteris), and so on for the
various organs of a plant - seeds, pollen, wood, roots, and sometimes even
short shoots and juvenile leaves are given different names because they are,
for the most part, always found in isolation.
In rare cases these various organs are
found connected to each other or connections can be made between them based on
their continued association in different formations (the latter is the case for
the Caytonia plant), in this instance a ‘whole plant hypothesis’ can be
produced. Such a hypothesis states that we know (or think) these different
organs are from the same single biological plant taxa, however, the various
organs retain their individual names, even when they are found connected
together. To make matters worse, even if you find an association between two
different morphospecies of organs, it does not necessarily mean that those same
organs in a different formation are part of the same plant (I can’t think of a
good example of this right now, but I will add an update to this when I do). A
further further complication is that in the case of leaves, you can have
multiple different leaf morphotaxa on the same plant (this is called
heterophylly, and it’s a pain-in-the-butt when trying to reconstruct
prehistoric plants).
Sorry,
this tangent ended up being very long, but it’s a complicated matter which has
major implications for palaeoartists, but it is often severely overlooked, and
it is crucial for understanding the reconstructions within this post.
*You Liar! Writing this section
(twice actually - had to condense it) has made me realise that I REALLY need to
do a whole blog post on this, with some diagrams to make understanding it
easier. Perhaps that should be my next project?
The Caytonia Plant
By far the most completely known caytoniale is the Caytonia-plant, which, as already mentioned, is composed of Caytonia, Caytonanthus and Sagenopteris. While a complete articulated plant is not known, it is thought that the Caytonia plant grew as a small to medium (or potentially large) deciduous forest tree (Fig. 9a, 11) (Harris, 1971; Retallack and Dilcher, 1988). This is based on several lines of evidence: woody stems attributable to Sagenopteris (through direct association of leaves) are thick in comparison to most shrubs (Fig. 5a), and the abundance of Sagenopteris leaves and associated reproductive organs in certain beds of the Ravenscar Group (Jurassic of Yorkshire) are indicative of large and/or abundant deciduous trees, which would drop their leaves seasonally en-masse. The presence of Caytonia and Caytonanthus in different stratigraphic layers has been used as evidence that these trees were dioecious (male and female organs on different individuals). It is possible that some variation in growth form existed across this group of plants. A small branching specimen possessing Sagenopteris-like leaves has been described from the Crato Formation (Fig. 5h) (Fanton et al., 2007); its stems are thinner than in S. phillipsi and S. colpodes var. large form (the aforementioned woody stems; Harris, 1971) and it possess smaller leaves, suggesting an overall smaller plant (even small young woody plants possess adult-sized leaves), but like its British counterpart it is also woody. For this plant I have suggested a single-stemmed small tree reconstruction, in-keeping with the growth form proposed for the Caytonia nathorstii plant (Fig. 9b), although it could also be a multi-stemmed shrub. The 2nd order branches of the Crato specimen are alternately arranged and porrect (angled upwards) and due to a lack of other information I have incorporated this into my Caytonia plant reconstruction, giving it porrect branches arranged roughly alternately on the main trunk (Fig. 11).
The woody stems of Sagenopteris
are covered in spirally arranged leaf scars which were raised up to 2mm from
the stem surface (Fig. 5a), this would have made the stems of the Caytonia
plant rather rough (Harris, 1971). This, and additional specimens (sometimes
with attached leaves; Fig. 5b) indicate that the leaves of Caytonia
plant were arranged spirally around the stem (Retallack and Dilcher, 1988).
Axillary buds, similar to the winter resting buds of many modern temperate
trees, suggest that branching in the Caytonia plant was spirally
arranged to some extent (and also reinforces the idea that this is a deciduous
tree), however higher order branching in the Crato specimen (Fig. 5h), and a
specimen of S. colpodes var. large form show that opposite branching was
also present. Stems covered in undeveloped scale-leaves and mature leaves (Fig.
5b) indicate that at least some high order branches and twigs were completely
enclosed in leaves. These bud-like short shoots (Fig. 5f) may have developed in
the branchlets with spirally arranged leaves (Fig. 5g1) or may have formed
clusters of leaves arranged spirally around the short shoot (Fig. 5g3). Leaves could
apparently also originate individually from branches, as indicated by the Crato
specimen (Fig. 5g2). In the Crato specimen branch nodes appear to possess ensiform
(sword-shaped) bracts (Fanton et al., 2007) and while their presence in
other Caytonia plant species is not known, they could speculatively be
included in reconstructions (Fig. 5e).
The development of Sagenopteris leaves is well documented (Barbacka and Bóka, 2000b; Elgorriaga et al., 2019) and is an interesting detail which could be included in some palaeoartistic reconstructions (Fig. 6). Adult Sagenopteris leaves are paripinnate (although they appear palmately compound due to the close arrangement of leaflet pairs; Retallack and Dilcher, 1988) and possess four elliptic leaflets (although individual specimens with three or five leaflets are not uncommon) which have a well defined midrib extending down most of the leaf (although it does not reach the apex) and anastomosing reticulate venation (I haven’t figured this venation here because it’s time consuming to draw, but there are plenty of images on Google which show it clearly). While their appearance is reasonably conservative across the different species, there is some variation, which should be taken into account when reconstructing Caytonia plants from specific formations.
Like the leaves, the different species
of reproductive organs (Caytonia and Caytonanthus) also vary
slightly, but such differences likely wouldn’t be noticeable in most pieces of
palaeoart. The female organs Caytonia are composed of small (<10mm)
and spherical cupules with a flattened lip obscuring the mouth of the pollen canal
and have a short thick pedicel which attaches to the axis of the fertile frond
(Fig. 5d). These cupules are arranged in subopposite pairs along the frond and
the mouth (and lip) are on the abaxial side (underside) of the frond. Scars on the
axis of some specimens indicates that cupules were likely shed when fully
developed (Harris, 1964; Retallack and Dilcher, 1988) and the abundance
of isolated seed in Caytonia-baring beds suggests that they were
released or dispersed from the cupules (Taylor et al., 2009). The male
microsporophylls, Caytonanthus, are composed of clusters of one to four broad
synangia arranged in opposite of subopposite pairs along a flattened axis (Fig.
5c). The reproductive fronds were (likely) arranged spirally around a thin axis
or branch (Retallack and Dilcher, 1988) and if this developed from short
shoot-like buds, as with the leaves, they may have formed long drooping ‘inflorescences’
(Fig. 5e), however without more complete specimens this remains speculative (but
tantalisingly beautiful). The cupules were almost certainly wind pollinated,
although the presence of caytoniale pollen in insect faeces indicates palynivory,
and potentially occasional insect pollination (Taylor et al., 2009); if
this latter mechanism was common then the cupules and synangia may have been
coloured to attract insects, although this is only speculation.
The Caytonia plant is thought
to have inhabited humid temperate forest environments with seasonally wet and
dry periods and occasional flooding (Retallack and Dilcher, 1988). It was
likely a moisture loving plant and this is reflected in its distribution, as it
is absent from arid equatorial regions of northern and central Gondwana (Prakash
and Das, 2017). Smaller species may have inhabited riparian habitats along the
banks of rivers or on floodplains.
The Reymanownaea Plant
The reconstruction for this plant should be the same as for the Caytonia plant, however it would differ of course in the shape of the cupules, which were kidney-shaped and lacked a prominent lip (Barbacka and Bóka, 2000a). The cupules were likely arranged along an axis in some way, but until more complete specimens are found this arrangement cannot be confirmed.
The Crato Seed Fern
While this taxon has not been formally described, a brief description of it (and accompanying photographs) are presented in Mohr et al. (2007). The leaves are small (~4cm), fern-like and imparipinnate, sometimes bipinnate, with lobes arranged suboppositely (Fig. 7a). The terminal lobe is consistently diamond-shaped and short. The venation is simple pinnate, and the leaf surface is covered in short hairs (Fig. 7b). The branches are woody and are described as trifurcating (although based on available photographs the branching is more complex; Fig. 7d). The cupules are found in direct attachment with these branches and appear very similar in structure to both Caytonia and Reymanownaea, although the lip is possibly toothed (Fig. 7c; Roberts, 2019). These cupules also appear to have been borne individually on pedicels directly from nodes on the branch, as opposed to being part of a fertile frond like in Caytonia. Given its woody nature and small leaves, I have reconstructed this plant as a small multi-stemmed shrub with a loose branch arrangement (Fig. 9c). More complete descriptions of this taxon are needed in order to assess its affinities and anatomy properly.
Unlike the Caytonia plant this
taxon has clear adaptations to living in an arid environment (small leaves with
hairs), which is unsurprising, considering the warm dry climate of the Crato
Formation. It was likely an uncommon element of a xeric transitional brushland.
The Ruflorinia/Ktalenia Plant
This
plant is a prime example of how a whole plant reconstruction sometimes doesn’t
provide much information about that the living plant would have looked like. An
association between the leave of Ruflorinia sierra and the female
cupules of Ktalenia circularis was displayed by Taylor and Archangelsky (1985).
The leaves of R. sierra are fern-like, up to 8cm long, imparipinnate
bipinnatified with oppositely arranged leaflets. The cupules of Ktalenia
circularis are near spherical (sometimes almost heart shaped) and
containing one or two large seeds with a small neck at their downward pointing apex,
which opens into a mouth (Fig. 8b). These cupules are arranged along a fertile
axis singly or in opposite or subopposite pairs and are surrounded at their
base by clusters of bracts (Fig. 8a). Fertile axes are attached to the bases of
sterile fronds (perhaps originating from axillary buds?). Unfortunately, it is
no known whether these fronds and reproductive axes attached to a woody stem or
not, and thus this taxon may have been herbaceous (as it was reconstructed by Ida Kalsta), however as all other known Caytoniales are woody in habit, it would be
most conservative to reconstruct this taxon as a small woody shrub or tree (Fig.
9d).
The Ruflorinia/Ktalenia plant was an understory species within a warm temperate forest; the cuticles of Ruflorinia show adaptations typical of xeric plants, suggesting that they lived in dry water stressed soils, however it has also been noted that such features can also occur in plants growing in wet environments (Carrizo et al., 2014).
 |
Fig. 10 My take on Tom Parker's speculative reconstruction of the Morrison Formation Hermanophyton with Sagenopteris elliptica leaves attached. One again the cosplaying T-posing idiot returns!!! |
Conclusion and a really cool plant!!!
So
in conclusion, this “short little blog which shouldn’t take too long to write
oh no I hope I have enough to say” has turned into something far longer and
more detailed than I intended, so if you’ve made it all the way to the end then
I congratulate you on your perseverance.
There’s only one thing which I really still want to talk about and that is a really cool speculative reconstruction of Hermanophyton originally created by Tom Parker (I did my own version too, based on his concept; Fig. 10), I mean, just look at this thing, it’s so aesthetically pleasing!!! The reason that I bring it up is two-fold: firstly, in Parker’s reconstruction he suggested that Sagenopteris (S. elliptica, as it’s from the Morrison Formation) leaves could have adorned this incredible 10m high by 12 cm wide trunk and this is a perfect example of how isolated plant organs can be reconstructed as complete different plants; and secondly, because Sagenopteris is a morphogenus, as opposed to a biological genus, vastly different and unrelated plant could have possessed Sagenopteris leaves, and since caytoniale reproductive organs are not present in the Morrison Formation, we cause be certain that these Sagenopteris leaves belonged to a Caytonia plant. While I really do love this reconstruction, I think it is unlikely that Hermanophyton has Sagenopteris leaves, instead, even in the absence of caytoniale reproductive organs, it is most conservative to reconstruct S. elliptica as a standard Caytonia plant (like that described throughout this blog post), but we should still keep such speculative ideas in mind, and at the end of the day, these are all working hypotheses and who knowns what weird and wonderful things the fossil record will throw at us next.
References
Barbacka, M., Bóka,
K. (2000a). A new
Early Liassic Caytoniales fructification from Hungary. Acta Palaeobotany, vol. 40,
iss. 2, pp. 85–111.
Barbacka, M., Bóka,
K. (2000b). The
stomatal ontogeny and structure of the Liassic pteridosperm Sagenopteris (Caytoniales)
from Hungary. International Journal of Plant Science, vol. 161, iss. 1, pp.
149-157.
Carrizo, M. A., Del
Fueyo, D. M., Medina, F. (2014). Foliar
cuticle of Ruflorinia orlandoi nov. sp. (Pteridospermophyta) from the Lower
Cretaceous of Patagonia. Geoboios, vol. 47, pp. 87-99.
Crane, P. R. (1985). Phylogenetic Analysis of Seed Plants and the
Origin of Angiosperms. Annals of the Missouri Botanical Garden, vol. 72, no. 4,
pp. 716-793.
Elgorriaga, A., Escapa,
I. H., Cúneo, N. R. (2019). Southern
Hemisphere Caytoniales: vegetative and reproductive remains from the Lonco
Trapial Formation (Lower Jurassic), Patagonia. Journal of Systematic
Palaeontology, vol. 17, iss. 17, pp. 1477-1495.
Fanton, J. C.,
Ricardi-Branco, F. S., Dilcher, D. L., Campos, A. C. A., Tavares, S. A. S.
(2007). Macrofóssil
inédito de Caytoniales na Formação Crato, Cretáceo Inferior, Bacia do Araripe,
NE, Brasil: Estudo preliminar. In: Carvalho, I. S., Cassab, R.C. T., Schwanke,
C., Carvalho, M. A., Fernandes, A.C.S., Rodrigues, M.A.C.R., Carvalho, M.S.S.,
Arai, M., Oliveira, E. Q. O. (Eds.), Paleontologia: Cenários da Vida (1 ed.).
Harris, T. M. (1933). A new member of the Caytoniales. New
Phytologist, vol. 32, iss. 2, pp. 97-113.
Harris, T. M. (1964). The Yorkshire Jurassic Flora 11.
Caytoniales, Cycadales & Pteridosperms. London; British Museum of Natural
History
Harris, T. M. (1971). The stem of Caytonia. Geophytology,
vol. 1, pp. 23 – 29.
Miller, I. M., Hickey,
L. J. (2010). The
Fossil Flora of the Winthrop Formation (Albian-Early Cretaceous) of Washington
State, USA. Part II: Pinophytina. Bulletin
of the Peabody Museum of Natural History, vol. 51, iss. 1, pp. 3-96.
Mohr, B. A. R.,
Bernardes-de-Oliveira, M. E. C., Loveridge, R. F. (2007). 19: The macrophyte flora of the Crato
Formation. In Martill, D. M., Bechly, G., Loveridge, R. F. (Eds.), The Crato
Fossil Beds of Brazil: Window into an Ancient World (pp.458-462). The Edinburgh
Building, Cambridge, CB2 8RU, UK: Cambridge University Press.
Prakash, N., Das, N.
(2017). First record
of microsporophyll genus Caytonanthus Thomas from Early Cretaceous beds of
South Rewa Gondwana Basin, India: Its evolutionary and palaeogeographical
significance. Island Arc, vol. 26, art. E12163.
Retallack, G. J., Dilcher,
D. L. (1988). Reconstructions
of Selected Seed Ferns. Annals
of the Missouri Botanical Garden, vol. 75, no. 3, pp. 1010-1057.
Roberts, E. A.
(2019). The Early
Cretaceous Crato Formation gymnosperms of north-east Brazil. (Doctoral thesis).
University of Portsmouth, Portsmouth.
Shi, G., Herrera, F.,
Herendeen, P. S., Clark, E. G., Crane, P. R. (2021). Mesozoic cupules and the origin of the angiosperm
second integument. Nature, Vol. 594, pp. 223-226.
Taylor, T. N.,
Archangelsky S. (1985).
The Cretaceous Pteridosperms Ruflorinia and Ktalenia and Implications on Cupule
and Carpel Evolution. American Journal of Botany, vol. 72, no. 12, pp.
1842-1853.
Taylor, T. N.,
Taylor, E. L., Krings, M. (2009).
Paleobotany: The biology and evolution of fossil plants (2nd ed.). 32
Jamestown Road, London NW 1 7BY, UK; Academic Press.
Thomas, H. H. (1925). The Caytoniales, a new group of
angiospermous plants from the Jurassic rocks of Yorkshire. Phylosophical Transactions
of the Royal Society B, vol 213, iss. 402-410.







Thanks for posting this blog...
ReplyDeleteLeaflet Distribution company Corby
Leaflet Distribution Company Cambridge
I need something like this but with gigantopteridales, please, I really love the way you draw each plant.
ReplyDelete